| Paste PDB data here |
|
Fixed: A picture display error has been fixed. Thanks to Alexey for the error report! (December, 2017).
Note: This page has been moved from ric.hi-ho.ne.jp (April, 2017).
Fixed: Alternative conformers and anisotropic temperature factor lines are now acceptable (July 27, 2010). Try Sample data 3.
Need a calculator for furanose? Try this.
Sample data 1
HETATM 7427 C1 LGC A1001 -22.300 -16.746 8.122 1.00 14.56 C
HETATM 7428 C2 LGC A1001 -22.433 -15.457 8.963 1.00 13.17 C
HETATM 7429 O5 LGC A1001 -23.134 -17.808 8.338 1.00 14.74 O
HETATM 7432 C3 LGC A1001 -23.170 -15.692 10.321 1.00 12.67 C
HETATM 7434 C4 LGC A1001 -24.378 -16.628 10.123 1.00 14.40 C
HETATM 7436 C5 LGC A1001 -23.853 -17.984 9.599 1.00 15.99 C
Sample data 2
HETATM 5164 C1 GAL A 701 24.929 6.486 27.826 1.00 35.16 C
HETATM 5165 C2 GAL A 701 25.427 7.148 26.520 1.00 33.43 C
HETATM 5166 C3 GAL A 701 25.873 8.603 26.728 1.00 32.63 C
HETATM 5167 C4 GAL A 701 24.849 9.386 27.561 1.00 34.51 C
HETATM 5168 C5 GAL A 701 24.545 8.626 28.849 1.00 35.63 C
HETATM 5169 C6 GAL A 701 23.520 9.351 29.706 1.00 34.95 C
HETATM 5170 O1 GAL A 701 25.996 6.224 28.665 1.00 39.00 O
HETATM 5171 O2 GAL A 701 26.523 6.406 26.001 1.00 27.19 O
HETATM 5172 O3 GAL A 701 26.043 9.235 25.461 1.00 27.16 O
HETATM 5173 O4 GAL A 701 23.648 9.550 26.819 1.00 32.17 O
HETATM 5174 O5 GAL A 701 23.998 7.327 28.528 1.00 36.64 O
HETATM 5175 O6 GAL A 701 23.858 9.281 31.084 1.00 35.65 O
Sample data 3
HETATM 3018 C2 ABGC A 403 14.424 34.676 29.808 0.65 6.77 C
ANISOU 3018 C2 ABGC A 403 791 1025 758 249 40 -117 C
HETATM 3019 C3 ABGC A 403 15.955 34.650 29.541 0.65 6.29 C
ANISOU 3019 C3 ABGC A 403 875 789 725 266 128 -16 C
HETATM 3020 C4 ABGC A 403 16.322 34.593 28.056 0.65 6.17 C
ANISOU 3020 C4 ABGC A 403 768 862 714 13 107 1 C
HETATM 3021 C5 ABGC A 403 15.090 34.392 27.191 0.65 6.73 C
ANISOU 3021 C5 ABGC A 403 776 768 1012 118 -2 -157 C
HETATM 3022 C6 ABGC A 403 14.556 32.942 27.110 0.65 6.40 C
ANISOU 3022 C6 ABGC A 403 896 857 678 -66 9 18 C
HETATM 3023 C1 ABGC A 403 13.514 35.268 28.769 0.65 6.62 C
ANISOU 3023 C1 ABGC A 403 782 945 789 -89 -38 3 C
HETATM 3024 O2 ABGC A 403 14.168 35.232 31.046 0.65 4.75 O
ANISOU 3024 O2 ABGC A 403 476 854 475 109 5 -72 O
HETATM 3025 O3 ABGC A 403 16.439 33.431 30.209 0.65 5.33 O
ANISOU 3025 O3 ABGC A 403 492 833 701 89 -136 -171 O
HETATM 3026 O4 ABGC A 403 16.991 35.846 27.653 0.65 6.19 O
ANISOU 3026 O4 ABGC A 403 790 772 789 65 65 -61 O
HETATM 3027 O5 ABGC A 403 14.034 35.297 27.467 0.65 6.98 O
ANISOU 3027 O5 ABGC A 403 794 1073 784 220 95 -33 O
HETATM 3028 O6 ABGC A 403 13.604 32.850 26.133 0.65 7.98 O
ANISOU 3028 O6 ABGC A 403 617 1380 1034 -129 -29 -13 O
Input lines must follow the PDB guideline.
Do not change the COLUMNS of atom name (13-16), x (31-38), y (39-46), and z (47-54) coordinates.
What's Cremer-Pople parameter?
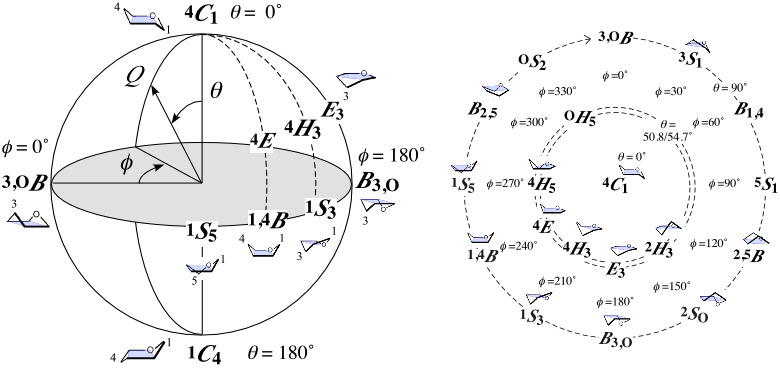
It is the puckering coordinate system of a six-membered pyranose ring [1]. Spherical polar representation (Fig. left; meridian angle φ, azimuthal angle θ, and radius Q) [2] and Stoddart (or "pseudorotational") diagram (Fig. right; projection of the sphere from the North Pole) are generally used. The radius Q means the magnitude of puckering, measuring the deviation from the perfectly flat six-membered ring (Q = 0).
The 38 basic conformations in the IUPAC nomenclature [3] are used to specify sugar ring conformations. They are 2 chair (θ = 0° or 180° at the Poles), 6 boat (θ = 90° on the Equator), 6 skew-boat (θ = 90° on the Equator), 12 envelope (θ = 54.7° in temperate zones), and 12 half-chair (θ = 50.8° in temperate zones) conformations. The Bérces-Whitfield-Nukada system that calculates the parameters as a linear combination of "ideal" basic conformations of "real" sugar rings is also described [4]. For force calculation during molecular simulation, I recommend the Hill-Reilly system [5].
References
-
D. Cremer & J. A. Pople: A General Definition of Ring Puckering Coordinates. J. Am. Chem. Soc. 97, 1354-1358 (1975) [doi]
-
G. A. Jeffrey & J. H. Yates: Stereographic representation of the Cremer-Pople ring-puckering parameters for pyranoid rings. Carbohydr. Res. 74, 319-322 (1979) [doi]
-
IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN) Conformational nomenclature for five and six-membered ring forms of monosaccharides and their derivatives, Recommendations 1980. Arch. Biochem. Biophys. 207, 469-472 (1981); Eur. J. Biochem. 111, 295-298 (1980); Pure Appl. Chem. 53, 1901-1905 (1981). [URL]
-
A. Bérces, D. M. Whitfield & T. Nukada: Quantitative description of six-membered ring conformations following the IUPAC conformational nomenclature. Tetrahedron 57, 477-491 (2001) [doi]: A nice server was once available at http://www.nrc.ca/ibs/6ring.html or http://www.sao.nrc.ca/ibs/6ring.html.
-
A. D. Hill & P. J. Reilly: Puckering Coordinates of Monocyclic Rings by Triangular Decomposition. J. Chem. Inf. Model. 47, 1031-1035 (2007) [doi] [PubMed]
About citation: No need to cite this URL because resources on The Internet are unstable. I would be happy if you acknowledge my name (Shinya Fushinobu) when you feel this site was helpful.
Thanks to Tony Hill and people of Reilly's lab at ISU (July-October, 2006).