 |
Name | State / change | Note |
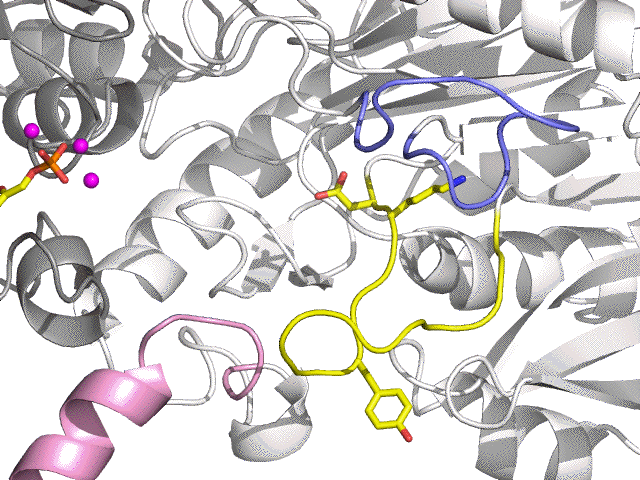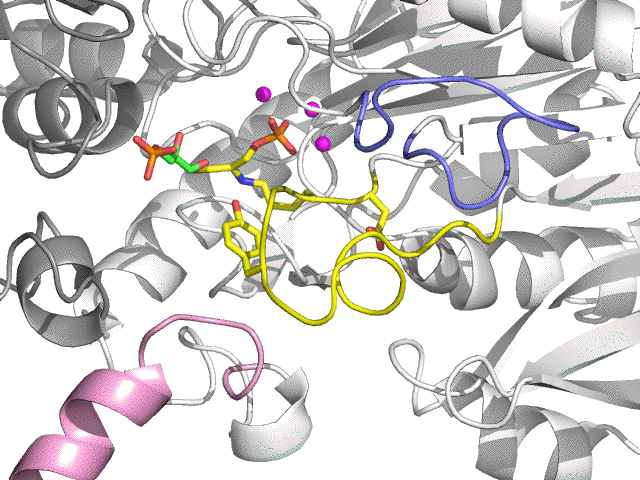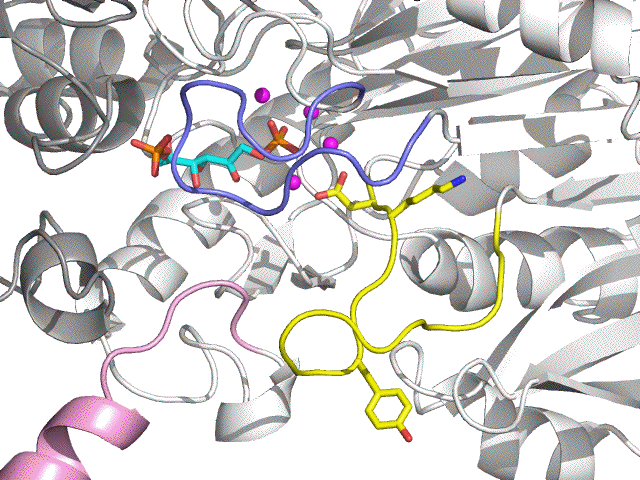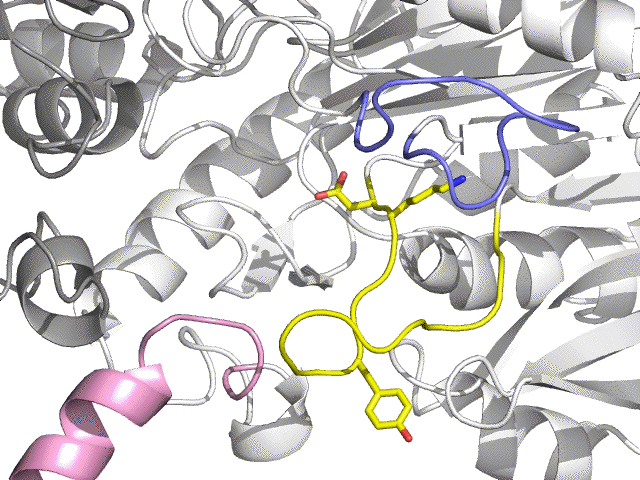
| E | Full open (Aldolase) |
||
| + DHAP (yellow) + 3 Mg2+ (magenta) |
Shiff Base loop (yellow) open | ||
| E:DHAP:3 Mg2+ | Aldolase form (DHAP Schiff Base) |
3RIM | |
| + GA3P (green) | |||
| E:DHAP:GA3P:3 Mg2+ | Aldolase form (Ternary complex) |
||
| Aldolase reaction | DHAP + GA3P |
||
| E:FBP:3 Mg2+ | Aldolase form (FBP complex) |
||
| Enzyme metamorphosis + 1 Mg2+ (magenta) |
Shiff Base loop (yellow) open Lid loop (blue) close C term. loop (pink) close |
||
| E:FBP:4 Mg2+ | Phosphatase form (FBP complex) |
1UMG | |
| Phosphatase reaction | FBP F6P (grey) + Pi (orange) |
||
| E:F6P:Pi:4 Mg2+ | Phosphatase form (product complex) |
||
| - F6P - Pi - 4 Mg2+ |
Lid loop (blue) open C term. loop (pink) open |
||
| E | Full open (Phosphatase) |
High quality QuickTime movie (11.9 MB) is here.