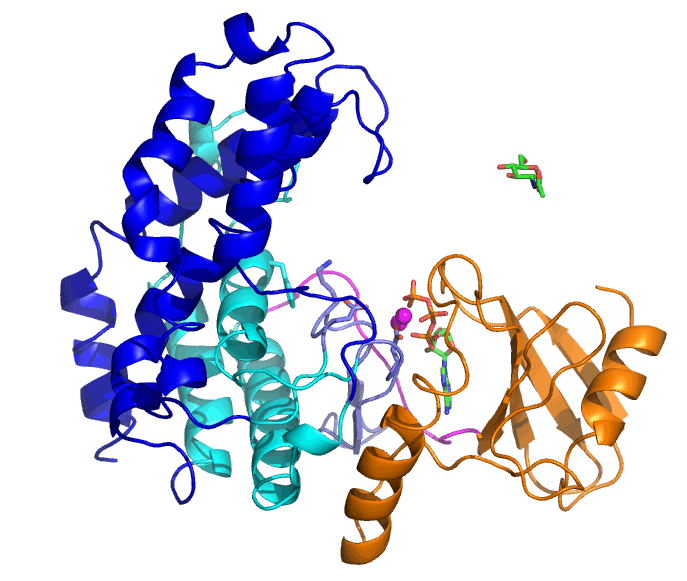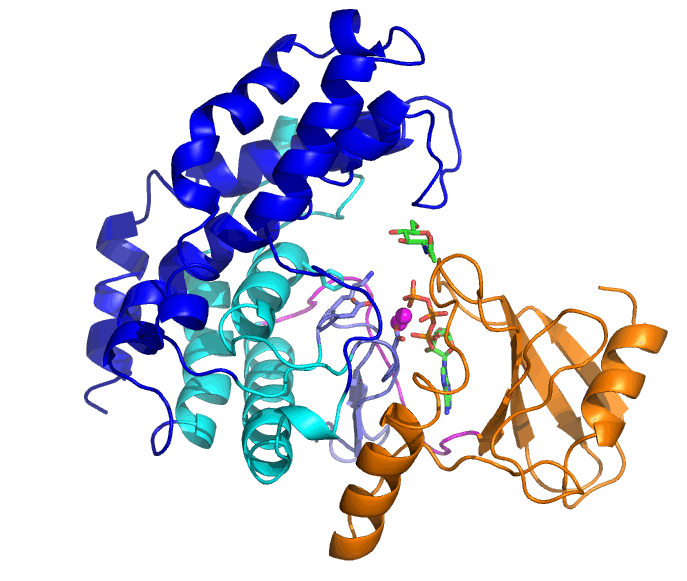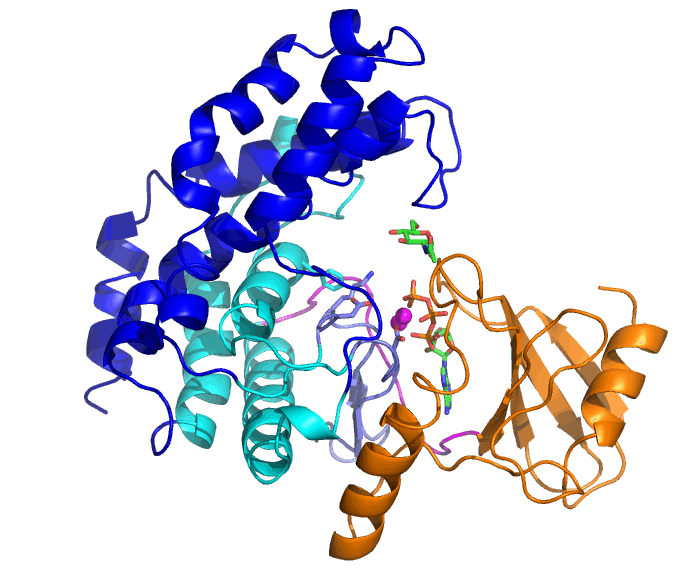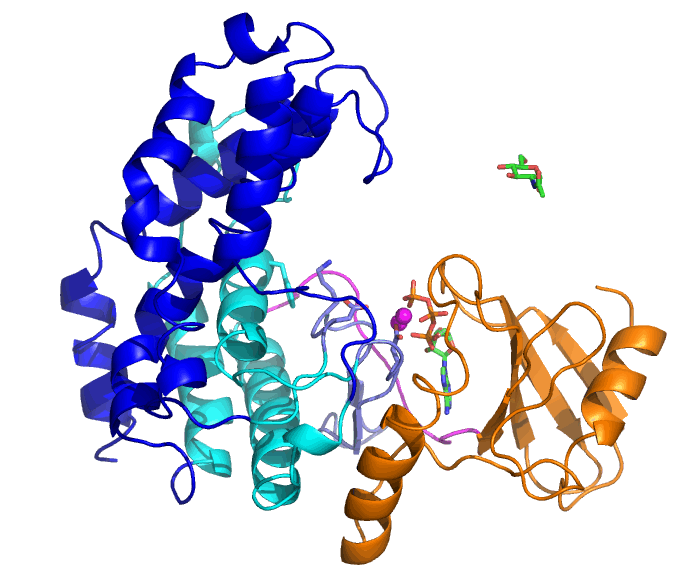
Bifidobacteria are living in the lower gastrointestinal tract of human and have various glycosidases that can degrade complex glycans. N-acetylhexosamine 1-kinase from Bifidobacteria (NahK) is involved in degradation of human milk oligosaccharides. Tha ability of NahK to produce 1-phosphate sugars has been applied to synthesis of oligosaccharides.

MPEG movie (1.1 MB) is here
The substrates of NahK are ATP, two Mg2+ ions, and a sugar (N-acetylglucosamine, GlcNAc, in this case). NahK moves from the open state that binds ATP and Mg2+ ions to the closed state by GlcNAc binding. A helical domain (blue and cyan) largely moves but the ATP-binding domain (orange) does not.
For details of this research, read our article on BBA.
M. Sato et al.
Open–close structural change upon ligand binding and two magnesium ions required for the catalysis of N-acetylhexosamine 1-kinase
BBA - Proteins Proteom. 1854 (5), 333–340 (2015)
M. Sato, T. Arakawa and S. Fushinobu